Introduction:
VEN is approved conditionally in the US in combination with HMA (azacitidine or decitabine) or low-dose cytarabine in newly-diagnosed AML patients (pts) ineligible for intensive chemotherapy due to age (≥75 years) or comorbidities. The Phase Ib clinical trial NCT02203773 (DiNardo et al. Blood 2019) and Phase III VIALE-A trial (NCT02993523; DiNardo et al. EHA 2020), demonstrated the clinical benefit of VEN+HMA, but frequent VEN dose modifications were observed due to the occurrence of cytopenias. This study aims to evaluate RW VEN+HMA response, treatment (Tx) duration, dose and schedule modifications in newly-diagnosed pts with AML treated predominantly in the community setting.
Methods:
This is a retrospective cohort study using the Flatiron Health electronic health record (EHR)-derived de-identified database; a nationwide database comprising patient-level structured (demographics, labs, visits and Tx administered in-network) and unstructured data (pathology reports, hospital discharges and physician notes, out-of-network and oral Tx), curated via technology-enabled abstraction (Flahavan et al. EHA 2020). Pts newly diagnosed with AML, age ≥18 years, and who initiated VEN+HMA within 30 days of diagnosis from June 1, 2018 to November 30, 2019 were evaluated. Additional variables related to VEN Tx were abstracted manually from EHR unstructured data: including Tx start/end dates, dose holds, schedule changes, dose changes (in mg) or discontinuation (d/c), and the reasons for these interruptions. Response to Tx was a measured "blast clearance" ≤5% from bone-marrow (BM) biopsy (unstructured) or peripheral blood (PB) (structured) data. RW complete response (CR)/CR with partial hematologic recovery (rwCR/CRh) was derived as ≤5% BM+PB blasts with partial hematologic count recovery within 14 days. Kaplan-Meier analyses were conducted to examine Tx duration of VEN+HMA Tx, with follow-up (f/u) to date of Tx d/c on VEN or censoring at the last EHR activity before data cut-off (February 29, 2020). VEN treatment cycles, derived based on the HMA dosing (28 days per cycle), were evaluated in a subset of pts with complete structured data on HMA administrations. Dosing intensity was calculated as dosed days as a percentage of total days from start to end of therapy or end of f/u.
Results:
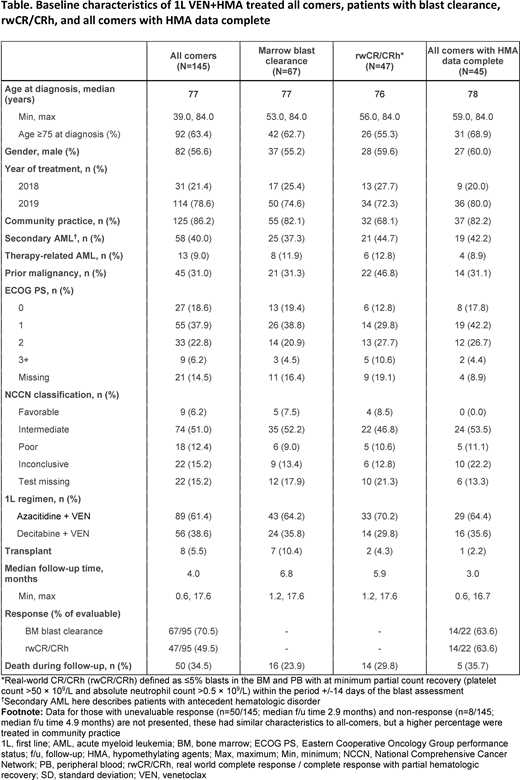
Overall 145 VEN+HMA pts (n=89, 61.4% received azacitidine combination) were included, median age at diagnosis was 77 years; 6% had Eastern Cooperative Oncology Group (ECOG) performance status ≥3; 9% had therapy-related AML (t-AML) and 40% had AML secondary to antecedent hematologic disorder (s-AML). Of the 95 pts with evaluable response data during the f/u period, 67 (71%) had BM blast clearance, and 47 (49%) had rwCR/CRh. At a median f/u of 4.3 months (range 0.6-17.7), 65 pts (45%) had not d/c VEN Tx at data cut-off. Data were not mature for overall survival analyses.
The subset of 45 pts with complete structured HMA data that were examined for VEN Tx scheduling was similar to the overall cohort; however, median f/u was slightly lower at 3.0 months (range 0.6-16.7). VEN initiation was not always concurrent with HMA initiation; 50% started within 2 days and 75% within 9 days of HMA initiation. Twenty-six pts (58%) had not d/c VEN at end of f/u; a median of 3 cycles was observed for all-comers in this time (range 1-15). Dose and schedule modifications (not mutually exclusive) occurring in cycles 1-3 included VEN dose changes (67%), d/c (36%), in-cycle dose interruption (24%) and cycle delay (22%). The most commonly cited reason for dose and schedule modifications was toxicity. Mean VEN dosing intensity overall was 77%; 86% prior to dose and schedule modification and 90% prior to dose change.
Conclusions:
This study is the largest RW data analysis of pts with AML treated with VEN+HMA to date. This RW cohort treated predominantly in the community setting differed from the VEN+HMA clinical trial: pts were older, had worse ECOG performance status and a higher proportion had s-AML. Despite this, in the evaluable RW VEN+HMA pts, high response was observed. The RW Tx duration analyses were limited by short f/u and RW VEN initiation was not always concurrent with HMA. Dose modifications were common early in Tx course. Further characterization of this cohort will have additional f/u and analyses on the timing of VEN dose modifications in relation to cytopenia management and drug-drug interactions.
Donnellan:PTC Therapeutics: Consultancy, Research Funding; Ryvu Therapeutics: Research Funding; Seattle Genetics: Consultancy, Research Funding; Takeda: Research Funding; TCR2 Therapeutics: Research Funding; AstraZeneca: Research Funding; Astex Pharmaceuticals: Research Funding; Amgen: Consultancy; Abbvie: Consultancy, Research Funding; Bellicum Pharmaceuticals: Research Funding; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; Celularity: Research Funding; CTI Biopharma: Research Funding; Daiichi Sankyo: Research Funding; Forma Therapeutics: Research Funding; Forty Seven: Research Funding; Genentech: Research Funding; H3 Biomedicine: Research Funding; Incyte: Research Funding; Janssen: Research Funding; Karyopharm Therapeutics: Research Funding; Kite Pharma/Gilead: Research Funding; MedImmune: Research Funding; Pfizer: Research Funding. Xu:F. Hoffmann-La Roche Ltd: Current Employment, Other: All authors received support for third party writing assistance, furnished by Scott Battle, PhD, provided by F. Hoffmann-La Roche, Basel, Switzerland.. Ma:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Jin:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Montez:F. Hoffmann-La Roche: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Choi:AbbVie: Current Employment, Current equity holder in publicly-traded company. Ku:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche: Current Employment, Current equity holder in publicly-traded company. Rajeswaran:F. Hoffmann-La Roche: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Ramsingh:NEKTAR: Current equity holder in publicly-traded company; F. Hoffmann-La Roche: Current equity holder in publicly-traded company; Exelixis: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months; Genentech, Inc.: Current Employment. Flahavan:Roche Products Ltd.: Current Employment; F. Hoffmann-La Roche: Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal